11 November 2014
Europe's forest fungi: diversity, distribution and fate
Fungi are responding to environmental change across Europe. Kew scientists Laura Martinez-Suz and Martin Bidartondo explain ambitious efforts to understand what is happening.
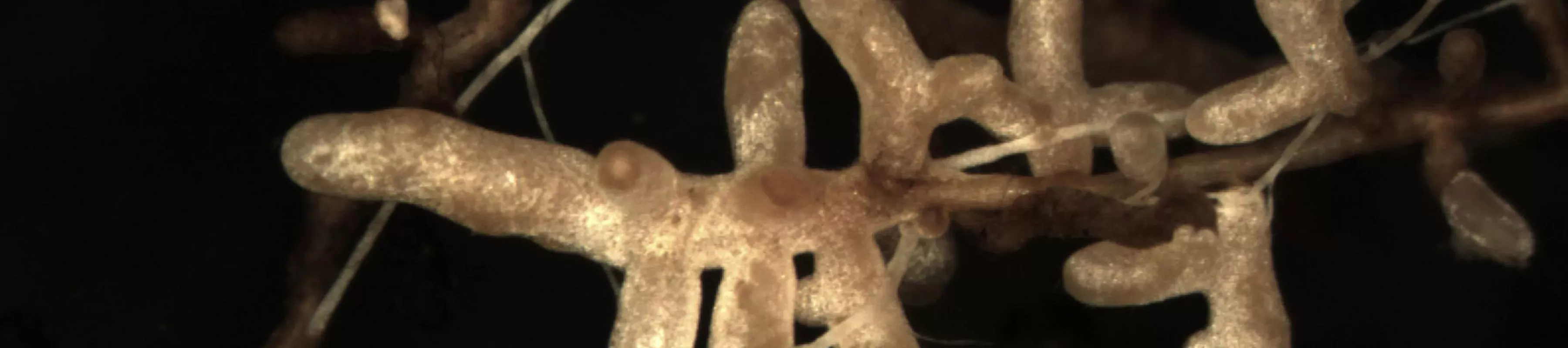
Plants lead a double life – their above-ground and below-ground worlds present radically different challenges and opportunities.
One has light and air, the other darkness, water and minerals. Shoots harness energy from light and air. Roots use the energy from the shoots to mine for water and minerals in the extraordinarily complex and fiercely competitive environment of the soil.
In other words, shoots are useless without water and minerals obtained by the roots, and roots are useless without energy from the shoots.
Working with underground partners
Shoots are much easier to study than roots: the dark soil is a barrier to observation, even for scientists using state-of-the-art technology. Thus, how roots manage to work within the soil did not start to be understood until relatively recently.
It turns out that while shoots have mastered the world above, the roots have contracted out to fungi most of their mining activities in the soil. Simply put, the filaments of fungi are thinner, longer, more branched and more interconnected than roots and root hairs, and fungi are far more diverse than plants (there are conservative estimates of at least six species of fungi for every plant species!).
Diversifying their tool-box with fungi has been a superb investment strategy for plants. In fact, the vast majority of plants do not simply have roots, they have mycorrhizas (or 'fungal roots') formed by fungal filaments growing into and out of roots.
In Europe's forests, nearly every root of every tree species is covered by a fungus. The fungus does the hard work of taking up water and nutrients from the soil, uses some for itself, and passes the rest to the root. As payment, the root gives some of the power it gets from the shoot to the fungus.
Different species of fungi have different mining abilities and demand different payment from roots. Different roots of the same plant can be colonized by different fungi and one fungus can colonize more than one root. This mutualistic symbiosis between plant roots and fungi is called ectomycorrhiza (which means 'outer fungus root').
We know some of these forest fungi from the wild mushrooms, truffles or crusts that they make when conditions are right for them to reproduce. Below ground, in the form of filaments and ectomycorrhizas, many of these fungi are very large and long-lived; some individuals can occupy several square metres of forest ground and live for decades.

Coping with big changes
Humans have been altering the environments in which plants and fungi live across the whole world, particularly since the Industrial Revolution.
For instance, we are releasing carbon and nitrogen from the Earth's crust by burning fossil fuels, and also releasing nitrogen from the atmosphere. Historical levels for carbon dioxide before the Industrial Revolution were about 300 ppm and nearly zero for atmospheric nitrogen deposition. The currently increasing levels are at about 390 ppm for carbon across the globe, and depending on the location, between 0 and 50 kilograms of nitrogen per hectare per year.
All this extra carbon and nitrogen is entering the biosphere. This is an unprecedented experiment on a global scale. How are our forests coping?
Ten years ago, members of the oldest, largest and most intensive forest monitoring network, the International Co-operative Programme on Assessment and Monitoring of Air Pollution in Forests (ICP Forests), became interested in how the partnership between trees and fungi was reacting to environmental change, and whether fungi could warn land managers of changes happening below ground before damage to shoots was evident.
A workshop led to a Natural Environment Research Council PhD student at Kew and Imperial College, Filipa Cox, publishing the first large-scale analysis of Scots pine forests and their mycorrhizal fungi.
The project was initially set up to study three forest monitoring plots in Britain, but Filipa managed to extend it to nine more in Germany. This was a huge effort - over 1,120 soil cores and 4,768 roots were analysed.
She found that nitrogen pollution was negatively affecting the diversity of fungi that trees could rely on; a few fungi appeared to thrive in disturbed conditions but most lost out. The tool-box for trees becomes less diversified in polluted areas.
This finding led to a Marie Curie fellowship at Kew and Imperial College London by Laura Martinez-Suz. Laura is an expert on oak mycorrhizas, and she managed to sample 22 oak monitoring plots across nine European countries - this time with 2,112 soil cores and 6,336 roots!
Laura recently published her major study showing that nitrogen pollution is also negatively affecting oak forests and at an even larger geographic scale. The fungi that lose out are those that can mine at long distances from the root and use organic nitrogen, the weaker short-distance inorganic nitrogen miners are winning. Her study is featured in the latest executive report of ICP Forests and was the highlight of a recent workshop held at Kew.
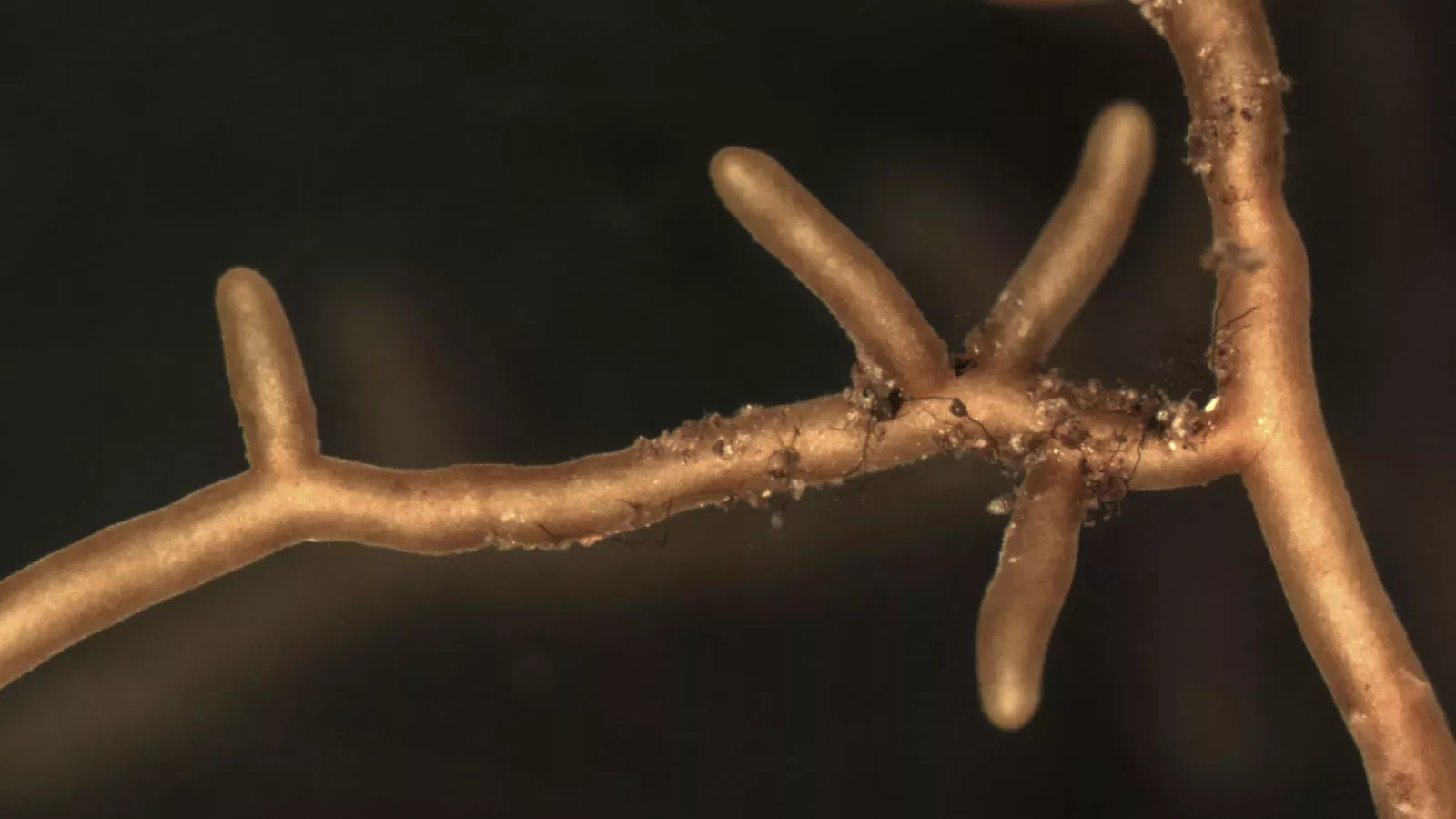
Where are the knowledge gaps?
Worryingly, fungi receive almost no protection under conservation policies.
Of the over 21,000 species that have been red-listed by the influential International Union for the Conservation of Nature (IUCN), only three are fungi and none of them are mycorrhizal.
In the words of natural historian Peter Marren in his 2012 book Mushrooms: 'We have only a very imperfect idea of the distribution and status of fungi, for field mycologists put most of their intellectual energies into identification. Hence, it is difficult to assess which species are genuinely rare or declining, and which are merely poorly recorded or simply do not fruit very often. Certain countries, most notably the Netherlands, Germany and Poland, have made creditable stabs at producing Red Lists (although only the Dutch have quantitative data to back it up). Elsewhere, the evidence is patchy.'
Fortunately, a new international initiative is finally working on a global Red List for fungi. Information from below ground, like Filipa's and Laura's, will be particularly useful.
Among others, back in 2007 ecologists Erik Lilleskov and Jeri Parrent called for unbiased, large-scale, DNA-based, data on mycorrhizal distributions, warning that not building up global data sets and models to define baseline diversity and distribution data of mycorrhizal fungi would be a lost opportunity in the face of rapid global change.

What are we doing?
Thanks to the Natural Environment Research Council, Sietse van der Linde and Bonnie Atkinson from Kew and Imperial College London are carrying out the most thorough study of forest mycorrhizal fungi yet undertaken.
So far, they have sampled 54 monitoring plots of spruce, pine and beech across most of Europe. Stay tuned for their discoveries regarding the diversity of the fungi they find, how and why fungal species are distributed across different forests, and how we can start to predict the future of fungi and forests in our changing world. This is a huge effort. Let us know if you want to help!
References
Suz, L.M., Barsoum, N., Benham, S., Dietrich, H.P., Fetzer, K.D., Fischer, R., García, P., Gehrman, J., Kristöfel, F., Manninger, M., Neagu, S., Nicolas, M., Oldenburger, J., Raspe, S., Sánchez, G., Schröck, H.W., Schuber, A., Verheyen, K., Verstraeten, A. & Bidartondo, M.I. (2014). Environmental drivers of ectomycorrhizal communities in Europe's temperate oak forests. Molecular Ecology, doi: 10.1111/mec.12947. Available online
Tse-Laurence, M.A. & Bidartondo, M.I. (2011). Mapping fungi from below ground: online genetic resources and ectomycorrhizal geographic distributions. iForest 4: 252-255.Available online
Cox, F., Barsoum, N., Bidartondo, M.I., Børja, I., Lilleskov, E., Nilsson, L.O., Rautio, P., Tubby, K. & Vesterdal, L. (2010). A leap forward in geographic scale for forest ectomycorrhizal fungi. Annals of Forest Science 67: 200. Available online
Cox, F., Barsoum, N., Lilleskov, E. &, Bidartondo, M.I. (2010). Nitrogen availability is a primary determinant of conifer mycorrhizas. Ecology Letters 13: 1103-1113. Available online
Cox, F., Barsoum, N., Lilleskov, E., Bidartondo, M.I. & Seidling, W. (2010). Mykorrhizierung von Kiefernwurzeln: Stickstoffverfügbarkeit als Einflussfaktor. AFZ-DerWald 65: 8-10.
Peay, K.G., Bidartondo, M.I. & Arnold, A.E. (2010). Not every fungus is everywhere: scaling to the biogeography of fungal-plant interactions across roots, shoots and ecosystems. New Phytologist 185: 878-882. Available online
Lilleskov, E.A. & Parrent, J.L. (2007). Can we develop general predictive models of mycorrhizal fungal community-environment relationships? New Phytologist, 174: 250-256. Available online
Marren, P. (2012). Mushrooms. The British Wildlife Collection no. 1. British Wildlife Publishing.




